
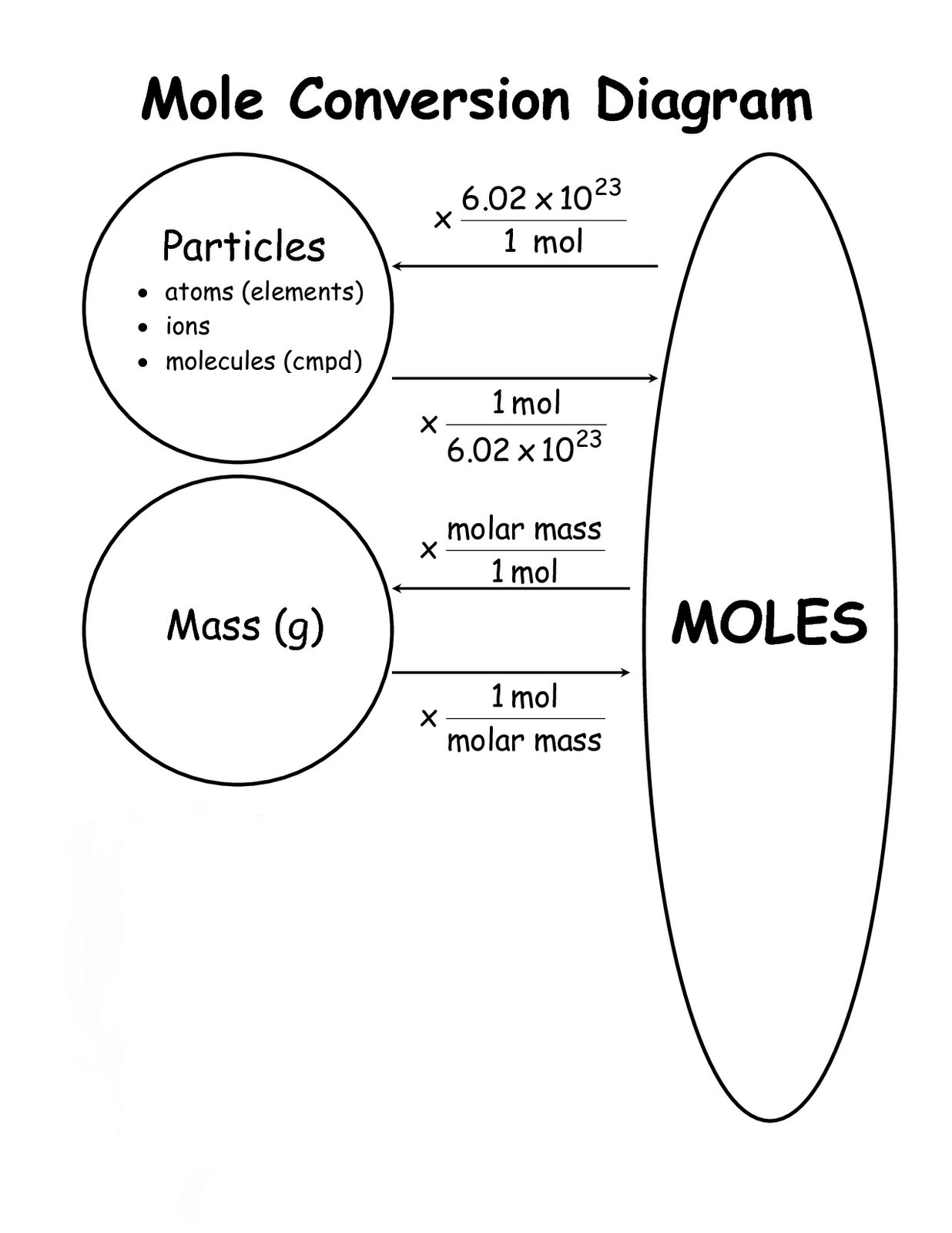
The molar mass of a substance depends not only on its molecular formula, but also on the distribution of isotopes of each chemical element present in it. For example, 100 g of water is about 5.551 mol of water. The number of moles of a substance in a sample is obtained by dividing the mass of the sample by the molar mass of the compound. Other methods include the use of the molar volume or the measurement of electric charge. For example, the molar mass of water is 18.015 g/mol. The amount of substance is given as the number of moles in the sample.įor most practical purposes, the numerical value of the molar mass expressed with the unit gram per mole is the same as that of the mean mass of one molecule of the substance expressed with the unit dalton. The molar mass of a substance is the ratio of the mass of a sample of that substance to its amount of substance. The mass of a substance is equal to its relative atomic (or molecular) mass multiplied by the molar mass constant, which is almost exactly 1 g/mol. Thus, common chemical conventions apply to the definition of the constituent particles of a substance, in other cases exact definitions may be specified. In yet other cases, such as diamond, where the entire crystal is essentially a single molecule, the mole is still used to express the number of atoms bound together, rather than a count of molecules. Thus the solid is composed of a certain number of moles of such particles. However, in a solid the constituent particles are fixed and bound in a lattice arrangement, yet they may be separable without losing their chemical identity. For example, a solution may contain a certain number of dissolved molecules that are more or less independent of each other. Usually the particles counted are chemically identical entities, individually distinct. The mole corresponds to a given count of particles. For example, 1 mole of MgBr 2 is 1 gram-molecule of MgBr 2 but 3 gram-atoms of MgBr 2.

The term gram-molecule was formerly used for "mole of molecules", and gram-atom for "mole of atoms". The concentration of a solution is commonly expressed by its molar concentration, defined as the amount of dissolved substance per unit volume of solution, for which the unit typically used is moles per litre (mol/L). For example, the chemical equation 2H 2 + O 2 → 2H 2O can be interpreted to mean that for each 2 mol dihydrogen (H 2) and 1 mol dioxygen (O 2) that react, 2 mol of water (H 2O) form. The mole is widely used in chemistry as a convenient way to express amounts of reactants and products of chemical reactions. The previous definition of a mole was the number of elementary entities equal to that of 12 grams of carbon-12, the most common isotope of carbon. The number of elementary entities in one mole is known as the Avogadro number, which is the approximate number of nucleons ( protons or neutrons) in one gram of ordinary matter.

For example, 10 moles of water (a chemical compound) and 10 moles of mercury (a chemical element), contain equal amounts of substance and the mercury contains exactly one atom for each molecule of the water, despite the two having different volumes and different masses. Depending on what the substance is, an elementary entity may be an atom, a molecule, an ion, an ion pair, or a subatomic particle such as an electron. The mole is defined as containing exactly 6.022 140 76 ×10 23 elementary entities. The quantity amount of substance is a measure of how many elementary entities of a given substance are in an object or sample. The mole (symbol mol) is the unit of amount of substance in the International System of Units (SI). For the mathematical technique, see Method of lines. In general, this is done by multiplying the total number of moles per unit of volume by the total volume.įor this example, the substance has a density of 500 moles per mL of substance and there are a total of 3 mL of substance."Nmol" redirects here.
#Moles to grams how to
How to calculate the number of atoms from moles?įirst, determine the total number of moles in the substance. Moles to Atoms DefinitionĬonverting moles to atoms is as simple as multiplying the number of moles by the 6.022 * 10^23 because by definition that is what a mole represents. To calculate the number of atoms from moles, multiply the number of moles by 6.0221415*10^23. The following formula is used to convert the total moles to total atoms. This calculator can also determine the number of moles given the atoms. The calculator will display the total number of atoms in those moles. Enter the total number of moles of a substance into the moles to atoms calculator.


 0 kommentar(er)
0 kommentar(er)
