
Therefore we have (still incorrect) 1s 22s 22p 63s 23p 63d 94s 2Ĭorrect Electron Configuration for Copper (Cu) Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation is written ( here is an explanation why). Note that when writing the electron configuration for an atom like Cu, the 3d is usually written before the 4s. Therefore the expected electron configuration for Copper will be 1s 22s 22p 63s 23p 64s 23d 9.
#As element number of electrons full#
After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d9. We now shift to the 4s orbital where we place the remaining two electrons.

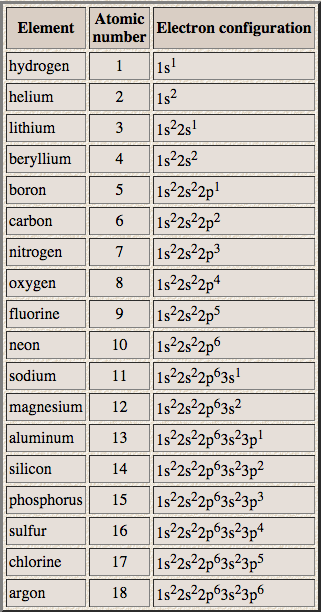
Since the 3s if now full we'll move to the 3p where we'll place the next six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. The p orbital can hold up to six electrons. The next six electrons will go in the 2p orbital. Since 1s can only hold two electrons the next 2 electrons for Copper go in the 2s orbital. In writing the electron configuration for Copper the first two electrons will go in the 1s orbital. The number of unpaired electrons in the last orbit of an element is the valency of that element.Video: Cu, Cu +, and Cu 2+ Electron Configuration Notation The ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). Since the last shell of an arsenic ion has eight electrons, the valence electrons of arsenic ions(As 3-) are eight. This electron configuration shows that the arsenic ion(As 3-) acquired the electron configuration of krypton and its last shell has eight electrons. The elements that receive electrons and form bonds are called anions.ĭuring the formation of a bond, the last shell of arsenic receives three electrons and turns into an arsenic ion(As 3-). The elements that have 5, 6, or 7 electrons in the last shell receive the electrons in the last shell during bond formation. Valence Electrons for Arsenic How many valence electrons does arsenic ion(As 3-) have? Therefore, the valence electrons of arsenic are five. The electron configuration shows that the last shell of arsenic has five electrons. The total number of electrons in a valence shell is called valence electrons. The last shell after the electron configuration is called the valence shell. The third step is to diagnose the valence shell. Step-3: Determine the valence shell and calculate the total electrons Therefore, the number of electrons per shell of arsenic is 2, 8, 18, 5. The electron configuration shows that the first shell of arsenic has two electrons, the second shell has eight electrons, the 3rd shell has eighteen electrons and the 4th shell has five electrons. We know that arsenic atoms have a total of thirty-three electrons. In this step, the electrons of arsenic have to be arranged. Step-2: Need to do electron configuration of arsenic That is, the arsenic atom has a total of thirty-three electrons. From the periodic table, we see that the atomic number of arsenic is 33. That is, we can finally say that there are electrons equal to the atomic number in the arsenic atom. Position of arsenic(As) in the periodic table Knowing the electron configuration in the right way, it is very easy to determine the valence electrons of all the elements. It is not possible to determine the valence electron without electron configuration.
The electron configuration is one of them. The valence electrons have to be determined by following a few steps. How do you calculate the number of valence electrons in an arsenic atom? The valence electrons determine the properties of the element and participate in the formation of bonds. The total number of electrons in the last shell after the electron configuration of arsenic is called the valence electrons of arsenic. The valence electrons are the total number of electrons in the last orbit(shell).
What are the valence electrons of arsenic? That is, an arsenic atom has a total of thirty-three electrons. Electrons equal to protons are located in a circular shell outside the nucleus. That is, the number of protons in arsenic is thirty-three. The atomic number is the number of protons. Protons and neutrons are located in the nucleus. The nucleus is located in the center of the atom. How many electrons and protons does arsenic have?

What are the valence electrons of arsenic?.How many electrons and protons does arsenic have?.


 0 kommentar(er)
0 kommentar(er)
